Why Quality Systems Define the Success of Lyophilised ODT Manufacturing
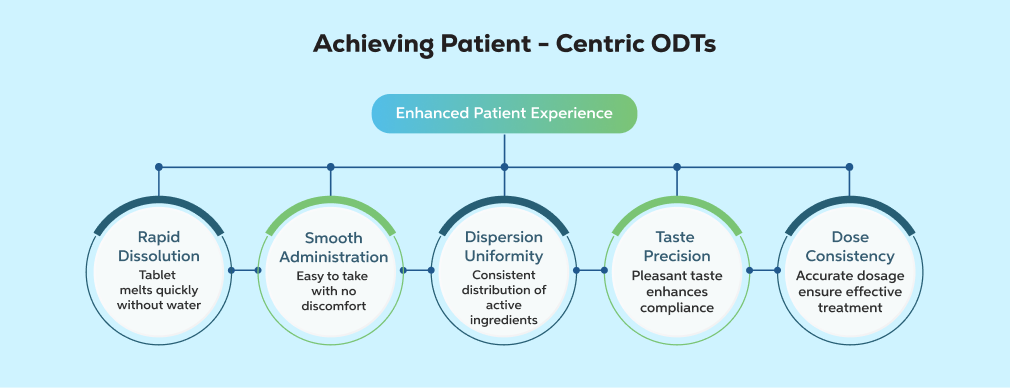
Lyophilised orally disintegrating tablets (ODTs), also known as oral lyophilisates, represent one of the most patient-centric oral solid dosage forms in contemporary pharmaceutical development.
The global orally disintegrating tablet market was valued at nearly USD 15 billion in 2024 and is projected to exceed USD 28 billion by 2033, reflecting sustained demand for patient-centric medications.
For the patient, the experience appears effortless; a unit dose that disperses within seconds in the oral cavity. Behind this apparent simplicity lies a manufacturing paradigm built on precision, process discipline, and structured quality control.
From a Quality standpoint, lyophilised ODTs are not inherently complex dosage forms. However, their lifecycle success depends on how systematically quality is engineered into material selection, process and equipment design, lyophilisation science, digital systems, and organisational culture.
Let’s explore a quality-centred view of lyophilised ODT manufacturing, integrating regulatory experience with commercial-scale production practices.
The Perception Gap in ODT Manufacturing
For patients, a lyophilised ODT delivers a near-frictionless experience, typically disintegrating in saliva within 3–10 seconds.From a manufacturing perspective, however, the quality framework supporting that experience is highly structured and unforgiving of deviation.
ODT technology has evolved from a niche paediatric convenience format into a strategic dosage form addressing:
- ● Adherence challenges
- ● Dysphagic and geriatric populations
- ● Patient usability requirements
- ● On-the-go administration needs
Despite this maturity, ODTs are often described as “complex dosage forms.” A more accurate framing is that they are precision-dependent dosage forms, where even small process drift produces immediately visible quality defects.
“ Compared to a normal tablet manufacturing line, our lyophilised ODT process has very few steps. It’s unique and very simple, but it requires a high degree of skill and zero tolerance for variability,” says Sreekumar G, Quality Assurance leader at InstaPill.
Regulatory Maturity and Inspection Readiness
Lyophilised ODT platforms today operate within well-defined regulatory expectations for:
- ● Process control
- ● Data integrity
- ● Lifecycle management
- ● Batch traceability
- ● Control strategy documentation
Facilities with multi-agency approvals (USFDA, EMA, MHRA, Health Canada, TGA) demonstrate that lyophilised ODTs are not experimental technologies but established, scalable dosage forms.
Regulatory success in this domain is not driven by episodic or reactive compliance activities, but by sustained platform consistency, scientific transparency, and demonstrable control of critical variables across the product lifecycle. Inspection readiness at InstaPill is continuous, driven by robust systems rather than a discrete event.
A Lean Process with No Room for Error
Compared with conventional tablet manufacturing, lyophilised ODT production involves fewer unit operations. However, each step functions as a critical quality gate.
Typical process flow:
- 1. Prepare dispersion
- 2. Fill into pre-formed cavities
- 3. Rapid freezing
- 4. Lyophilisation
- 5. Pack and release
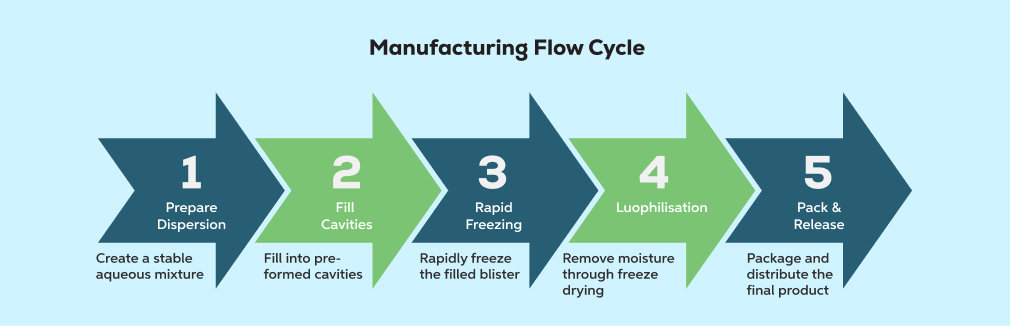
Because the process architecture is lean, there is limited capacity to buffer errors. Loss of control at any stage is rapidly reflected in:
- ● Content uniformity
- ● Disintegration performance
- ● Mouthfeel
- ● Structural integrity
- ● Sensory acceptability
The manufacturing design, therefore, emphasises precise execution, well-defined work instructions, and systematically validated operating ranges. From a Quality lens, each unit operation is therefore treated as a validated control point within an integrated control strategy.
The first quality gate: Designing quality from raw materials to QbD
From a quality perspective, the right selection of vendors and materials will play a significant role in product quality. While assay specifications may align with those of conventional oral solids, even minor variations in dispersion behaviour during filling can result in significant intra-batch variability.
Consequently, QbD for lyophilised ODTs effectively starts with understanding and controlling material behaviour under real processing conditions, rather than at the point of end-product testing.
In lyophilised ODT manufacturing, quality is not “inspected in” at the end of the process; it is designed in from the earliest stages, beginning with decisions on which materials are qualified for use. InstaPill’s Quality philosophy aligns with ICH Q8 principles of QbD, focusing on the proactive identification and control of Critical Material Attributes (CMAs).
Mapping CQAs to CPPs: Where Quality Becomes Measurable
After material qualification, the quality focus shifts to mapping:
Critical Quality Attributes (CQAs) → Critical Process Parameters (CPPs)
Key CQAs include:
- ● Assay and content uniformity
- ● Disintegration time
- ● Dissolution performance
- ● Residual moisture
- ● Impurity profile
- ● Structural integrity
These are directly influenced by CPPs such as:
- ● Mixing intensity and duration
- ● Dispersion hold time stability
- ● Fill accuracy
- ● Filling uniformity
- ● Lyophilisation temperature/time profiles
Validated parameter ranges create proven acceptable operating windows. Operating within these windows enables predictable quality outcomes and evidence-based batch release decisions.

Lyophilisation: The Silent Determinant of Patient Experience
Within a Quality by Design framework, the lyophilisation process is designed and controlled to ensure consistent attainment of CQAs of oral lyophilisates, including organoleptic performance. Freezing kinetics and primary and secondary drying parameters are defined as Critical Process Parameters CPPs based on an understanding of heat and mass transfer, pore structure formation, and moisture control.
Robust cycle design, supported by process characterization and representative sampling across the lyophiliser chamber, ensures a uniform thermal history and moisture distribution across the batch.
This science- and risk-based control strategy minimizes variability, prevents structural failure or delayed disintegration, and demonstrates that consistent patient-relevant performance is built into the process, aligning with regulatory expectations for product and process robustness.
Sampling Strategies That Reflect Real Variability
Lyophilised ODT quality systems reject assumptions of inherent uniformity inside the lyophiliser chamber. Instead, sampling plans are designed to capture:
- ● Shelf-to-shelf variability
- ● Quadrant variability
- ● Thermal gradient effects
- ● Moisture distribution differences
Sampling is intentionally multi-location across each load. A cycle is considered successful only when uniform performance is demonstrated across the entire chamber, not inferred from a single sampling point.
This spatially aware sampling reduces the risk of undetected gradients and supports batch-wide confidence in quality.
Digital Quality Systems as a Structural Advantage
Digital QA infrastructure strengthens control and traceability across the product lifecycle. Integrated digital systems typically include:
- ● Electronic batch records
- ● Electronic logbooks
- ● Digital QMS
- ● Material management systems
- ● Document management
- ● Training management platforms
- ● Facility management systems
Alignment with ALCOA+ data integrity principles ensures:
- ● Traceability
- ● Version control
- ● Audit readiness
- ● Reduced transcription error
- ● Faster deviation response
Digitalisation converts quality oversight from retrospective review into real-time governance.
Quality Culture: Accountability at Every Level
The long-term sustainability of any quality system is ultimately determined by organisational culture. At InstaPill, each batch is viewed as a product that individual teams are prepared to personally stand behind, creating a culture of ownership rather than mere procedural adherence.
Quality is positioned as an integrated, cross-functional partner in product realisation throughout the life cycle. This is managed as a collaborative, science-based development exercise, often involving multiple formulation iterations to reconcile clinical requirements with patient acceptability. In this model, consistency is deliberately engineered and continuously reinforced, rather than assumed.
Precision as the Future of ODT Manufacturing
The future of lyophilised ODT manufacturing belongs to organisations capable of delivering precision at scale under rising regulatory and patient expectations.
Platform strengths will be defined by:
- ● Material science integration
- ● QbD-driven development
- ● Automated manufacturing
- ● Digital QA systems
- ● Structured control strategies
- ● Culture of operational discipline
Looking ahead
Precision may not be visible to the patient, but it remains the defining link between manufacturing practice, regulatory confidence, and patient experience.
As quality expectations continue to rise, the companies that succeed will be those that treat quality as an everyday discipline, not an event, and a mindset central to InstaPill’s culture and CDMO approach.
“Skill, discipline, and transparency drive quality here. The technology works because the teams behind it treat precision as non-negotiable,” says Sreekumar G.
Precision may not make headlines, but in ODT manufacturing, it’s the only story that matters - and InstaPill writes it well!
Key Takeaways
- ● Precision drives product performance. Lyophilised ODT quality depends on tight control of materials, process parameters, and lyophilisation cycles, with very low tolerance for variability across unit operations.
- ● Quality is designed into the process from the start. Material qualification, Critical Material Attribute (CMA) control, and QbD-driven development establish the foundation for consistent batch outcomes and lifecycle reliability.
- ● CPP–CQA mapping enables predictable manufacturing Validated relationships between Critical Process Parameters and Critical Quality Attributes support measurable control strategies and evidence-based batch release decisions. Digital quality systems strengthen inspection readiness.
- ● InstaPill’s platform integrates QbD, digital QA, and controlled lyophilisation science this structured manufacturing and quality framework supports scalable, inspection-ready production of lyophilised ODTs with consistent patient-relevant performance.
References:
- Straits Research. (n.d.). Orally disintegrating tablet market.
- Pharma Excipients. (n.d.). Orally disintegrating tablets (ODTs).
- Žiberna, M. B., & Grabnar, P. A. (2023). Application of Quality by Design principles to the development of oral lyophilizates containing olanzapine. Pharmaceutics, 15(7).
- Ghourichay, M. P. (2021). Formulation and quality control of orally disintegrating tablets. Journal of Pharmaceutical Sciences, 1–xx.
- Costa, J. S. R., et al. (2019). A mini-review on drug delivery through wafer technology: Formulation and manufacturing of buccal and oral lyophilizates. International Journal of Pharmaceutical Sciences, 1–xx.
- Pardeshi, S. R. (2023). Process development and quality attributes for the freeze-drying of pharmaceuticals: Applications of Quality by Design. International Journal of Pharmaceutical Sciences, 1–xx.

