Introduction
Non-adherence is a global crisis: the World Health Organisation estimates that one in two patients does not follow their prescribed therapy, leading to chronic disease treatment failures and an estimated $100–300 billion in excess healthcare spending each year.
For payers, the burden is unsustainable. For regulators, the solution is increasingly clear: demand medicines that are clinically effective, acceptable, usable, and aligned with patient behaviour.
This shift marks the rise of what can be called the Adherence Economy. In this new landscape, competitive advantage is defined not simply by How the drug functions or price, but by how well a therapy integrates into the patient’s daily life.
Taste, swallowability, convenience, and speed of action have moved from the margins of formulation design to the centre of regulatory review and market access decisions.
Orally disintegrating tablets (ODTs) sit at the heart of this transformation. By eliminating the need for water, reducing swallowing effort, and delivering a smoother sensory experience, ODTs directly address the leading causes of poor adherence in paediatric, geriatric, and even psychiatric populations.
What was once viewed as a “nice-to-have” format has become a strategic tool in reducing treatment failure, improving real-world outcomes, and unlocking payer and regulatory support.
Patient Realities: When Tablets Fail
The dominance of oral solid dosage (OSD) forms in drug pipelines, representing 84% of medications in the market, collides with a stark behavioural reality:
Surveys show that nearly 50% of patients experience dysphagia or discomfort when swallowing tablets or capsules. Pill-specific dysphagia affects ~14%. Around 18% had difficulty swallowing tablets without training. Patients frequently resort to splitting, crushing, or dissolving tablets-behaviours that compromise safety, alter pharmacokinetics, and risk subtherapeutic dosing.
Many discontinue treatment altogether, particularly in chronic or preventive therapies where immediate benefits are less visible.
These realities expose a fundamental gap: drug design has historically prioritised manufacturability and stability, while overlooking the lived experience of patients.
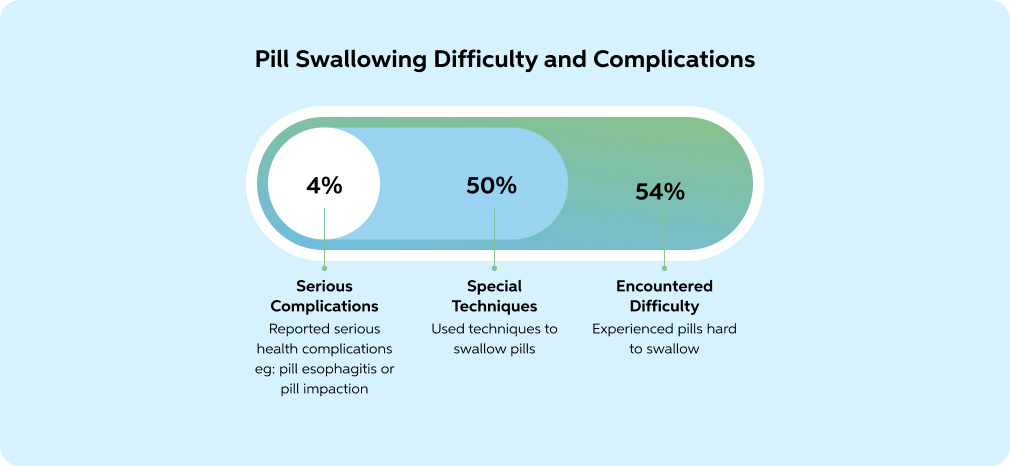
Adapted from: Fields, Jeremy, et al. "Pill Properties That Cause Dysphagia and Treatment Failure." Current Therapeutic Research, vol. 77, 2015, pp. 79-82, https://doi.org/10.1016/j.curtheres.2015.08.002.
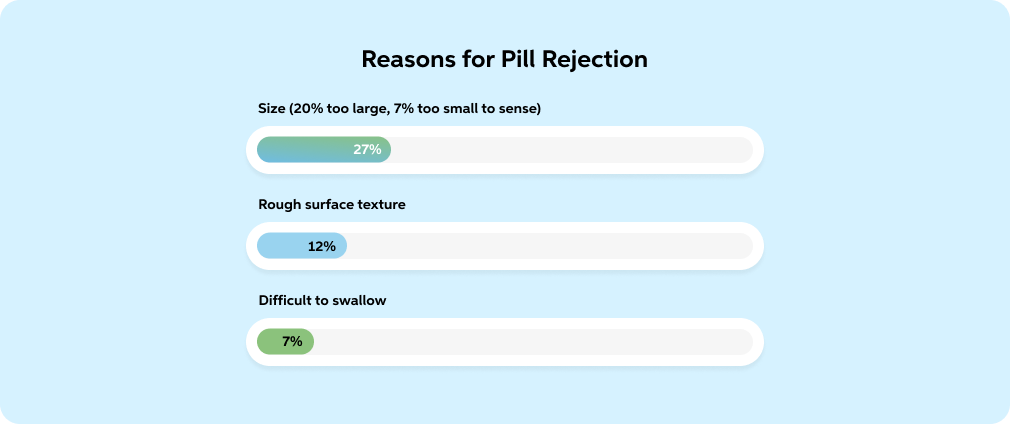
Adapted from: Fields, Jeremy, et al. "Pill Properties That Cause Dysphagia and Treatment Failure." Current Therapeutic Research, vol. 77, 2015, pp. 79-82, https://doi.org/10.1016/j.curtheres.2015.08.002.
But even when tablets are not the barrier, other dosage forms introduce challenges:
“When medicines are given as liquid solutions/suspensions, measuring a precise 5 mL dose is nearly impossible — caregivers often end up giving 4.5 or 5.5 mL instead. That inaccuracy can affect treatment outcomes. Most suspensions or solutions require preservatives because they’re in aqueous form. That raises long-term safety and tolerability concerns, especially in children and elderly patients,”.
“For years, many formulations relied on gelatin as a core excipient. But gelatin is derived from animal sources, which means you can’t claim vegan or vegetarian suitability — a growing concern in today’s market,” he continues — Balaji Sathurappan, InstaPill.
ODTs as a Strategic Solution
Orally disintegrating tablets (ODTs) from InstaPill have emerged as a response to this gap. By dispersing in seconds—without needing water- they eliminate the barrier of swallowability. Advances in formulation science, including effective taste masking, smoother mouthfeel, and faster onset of action, have elevated ODTs from niche formats to mainstream solutions.
“Patients barely realise they are taking medicine — the tablet melts instantly, leaving no grittiness behind.” — Balaji Sathurappan, InstaPill
Critically, ODTs are not simply about convenience. They are adherence tools:
- ● They reduce dose skipping in populations with dysphagia.
- ● They improve safety by removing the need for patients to modify dosage forms.
- ● They enhance quality of life by aligning therapy with patient preference.
Regulators and payers elevate patient experience as part of the therapeutic value equation. The FDA’s 2019 Guidance on Orally Disintegrating Tablets (ODTs) and the EMA’s reflection papers on patient acceptability highlight taste, swallowability, and ease of administration as elements that must be addressed in submissions—especially for paediatric and geriatric indications.
At the same time, value-based care models are pushing payers to seek therapies that improve long-term adherence and reduce downstream costs. Increasingly, market access negotiations factor in not only clinical efficacy but also how well a drug can be taken consistently and correctly by patients in the real world.
InstaPill’s Role in Encouraging Medication Adherence
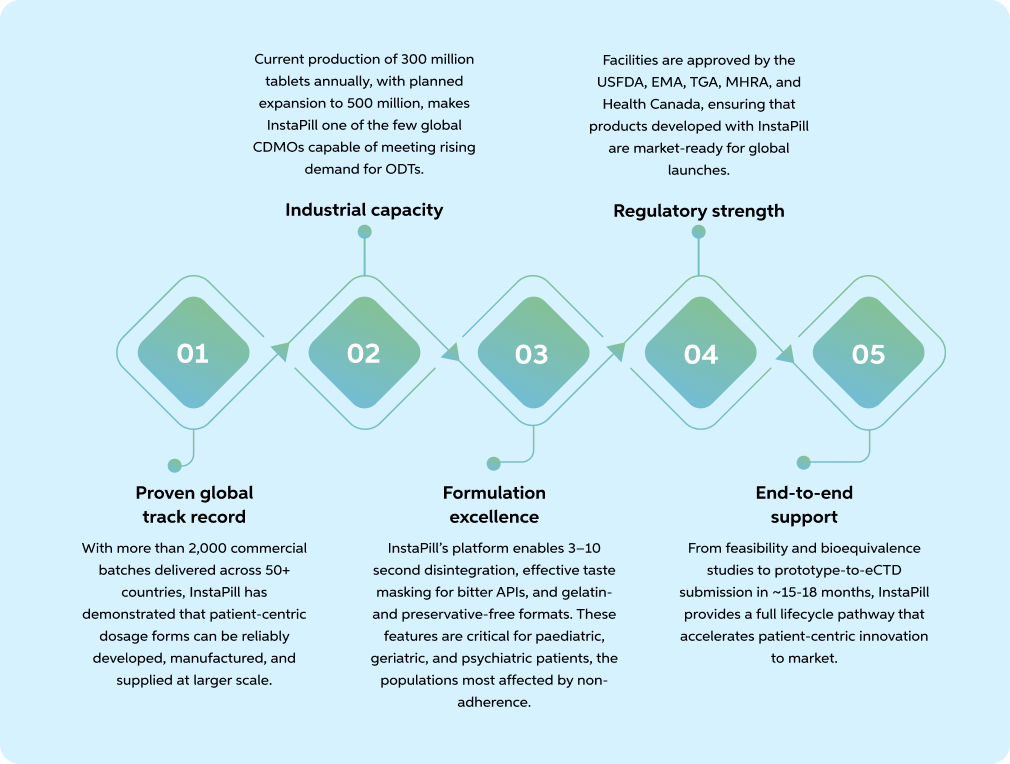
While ODTs are widely recognised as a solution to adherence challenges, scaling them from concept to commercial reality requires scientific expertise, regulatory credibility, and industrial capacity.
This is where InstaPill has established itself as a differentiated partner.
“Our technology is among the finest in efficiently masking the taste of active ingredients. Since more than 90% of APIs are bitter/unpleasant or cause throat irritation, we can completely eliminate that bitterness using InstaPill`s platform technology,” — Balaji Sathurappan, InstaPill
In Summary
For pharmaceutical companies navigating the dual pressures of improving outcomes and competing in crowded markets, InstaPill offers a proven platform to transform adherence from a liability into a market advantage.
Key Takeaways
- 1. Non-adherence is costly and widespread: Nearly half of all patients fail to take medicines as prescribed, driving chronic disease treatment failures and adding an estimated $100–300 billion annually in avoidable healthcare costs.
- 2. Patient experience is now a regulatory and payer priority: Taste, swallowability, convenience, and speed of action have moved to the centre of drug evaluation, especially in paediatric and geriatric populations.
- 3. Traditional dosage forms fall short: Large tablets, imprecise liquid dosing, preservatives, and presence of gelatin create barriers to adherence and limit patient choice.
- 4. ODTs provide a patient-centric alternative: By disintegrating in seconds, eliminating the need for water, and masking bitterness, ODTs improve adherence, safety, and quality of life for vulnerable patient groups.
- 5. InstaPill delivers ODTs at a global scale: With >2,000 batches across 50+ countries, 300 million+ tablets annually (expanding to 500 million), and a fully compliant, vegan, preservative-free platform, InstaPill enables pharma partners to turn adherence into a competitive advantage.
References:
- Adams, R., Crisp, D. A., & Thomas, J. (2024). The psychological impacts of pill dysphagia: A mixed methods study. Dysphagia, 39(6), 1202–1212.
- Achterbosch, M., et al. (2025). Clinical and economic consequences of medication nonadherence: A review of systematic reviews. Frontiers in Pharmacology, 16, 1570359.
- Centers for Disease Control and Prevention. (2017). Vital signs: Prescription opioid pain reliever use during pregnancy — 34 U.S. jurisdictions, 2019. MMWR. Morbidity and Mortality Weekly Report, 66(45), 1245–1251.
- Fields, J., Go, J., & Mann, R. (2015). Pill properties that cause dysphagia and treatment failure. Current Therapeutic Research, 77, 79–82.
- Harvard Health Publishing. (2019). When pills pose problems. Harvard Medical School.
- Lam, W. Y., & Fresco, P. (2015). Medication adherence measures: An overview. BioMed Research International, 2015, 217047.
- PharmaSource. (2024). Oral solid dosage (OSD) contract manufacturing market report.
- Sato, S., Sasabuchi, Y., Okada, A., et al. (2025). Do orally disintegrating tablets facilitate medical adherence and clinical outcomes in patients with post-stroke dysphagia? Dysphagia, 40(2), 381–387.

